A groundbreaking study published on December 8, 2023, by researchers at Sanford Burnham Prebys, in collaboration with institutions such as the University of Southern California, Juntendo University in Japan, Tokyo University of Pharmacy and Life Sciences, and the State University of New York, has unveiled a significant molecular connection between vitamin B12 and multiple sclerosis (MS). The study sheds light on the crucial involvement of astrocytes, non-neuronal glial cells in the brain, and the brain’s vitamin B12 carrier protein, transcobalamin 2 (TCN2), in this link.


The researchers, led by Jerold Chun, M.D., Ph.D., and Yasuyuki Kihara, Ph.D., discovered that the MS drug fingolimod plays a pivotal role in enhancing the brain’s B12 uptake. This revelation opens up exciting possibilities for improving MS treatments by mitigating neuroinflammation and neurodegeneration, key factors in the progression of this chronic disease.
One of the key findings of the study is that fingolimod, an FDA-approved MS drug, regulates B12 communication pathways within astrocytes, leading to an enhanced absorption of B12 in the brain. Importantly, lower levels of a B12 receptor known as CD320 or dietary B12 restriction were identified as exacerbating MS in animal models, emphasizing the critical role of B12 in managing the disease.
Jerold Chun emphasized the potential for brain-targeted B12 formulations, stating, “Augmenting brain B12 with fingolimod or potentially related molecules could enhance both current and future MS therapies.” The study demonstrates the intricate relationship between TCN2 and fingolimod, showing how this interaction reduces neuroinflammation and potentially slows neurodegeneration in MS patients.
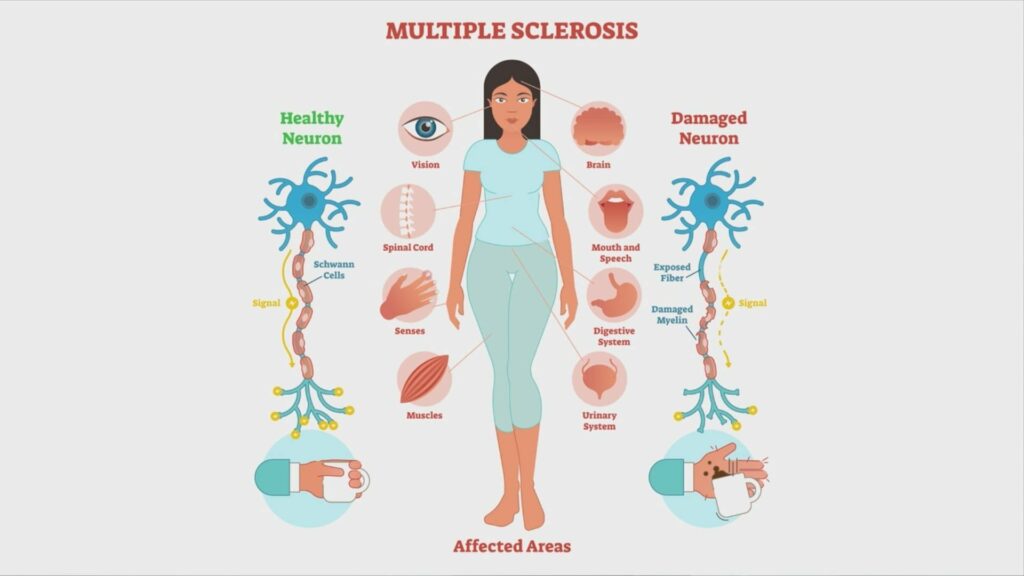
The research not only underscores the importance of B12 supplementation in the context of MS but also paves the way for innovative therapies targeting neuroinflammatory and neurodegenerative diseases. Fingolimod’s ability to correct impaired astrocyte-B12 pathways offers hope for improving MS treatments, and the study suggests that other S1P receptor modulators on the market may access similar CNS mechanisms.
In essence, the study not only deepens our understanding of the molecular connections between B12 and MS but also offers a promising avenue for the development of more effective therapies for a spectrum of neuroinflammatory and neurodegenerative conditions. These findings could potentially reshape the landscape of treatments for diseases affecting the central nervous system, offering renewed hope to individuals grappling with the challenges posed by MS and related conditions.