An important trial is underway in the UK for the world’s first “personalised” mRNA vaccine against melanoma, the deadliest form of skin cancer. Steve Young, 52, from Stevenage, Herts, who underwent surgery to remove a melanoma growth from his scalp last August, is among the initial recipients of the vaccine. “It is designed to help his immune system recognize and eliminate any remaining cancerous cells, with the hope that his cancer will not return,” he explained.
The vaccine, known as mRNA-4157 (V940), employs the same technology as current COVID-19 vaccines and is currently undergoing final-stage Phase III trials. Doctors at University College London Hospitals (UCLH) are administering it alongside another drug, pembrolizumab or Keytruda, which aids the immune system in targeting cancer cells.
“The combined treatment, developed by Moderna and Merck Sharp and Dohme (MSD), is not yet available outside of clinical trials in the NHS. Trials are also underway in other countries such as Australia to gather more evidence,” Young stated.
“This vaccine is tailored to each individual patient, matching the unique genetic signature of their tumour. It prompts the body to produce proteins or antibodies that specifically attack cancer cells,” explained Dr. Heather Shaw, an investigator at UCLH. “This level of customization is unprecedented and holds promise not only for melanoma but also for other cancers such as lung, bladder, and kidney tumors.”
“The UK arm of the international trial aims to enrol at least 60-70 patients across eight centres, including London, Manchester, Edinburgh, and Leeds. Participants must have undergone surgical removal of their high-risk melanoma within the last 12 weeks to qualify,” Young added.
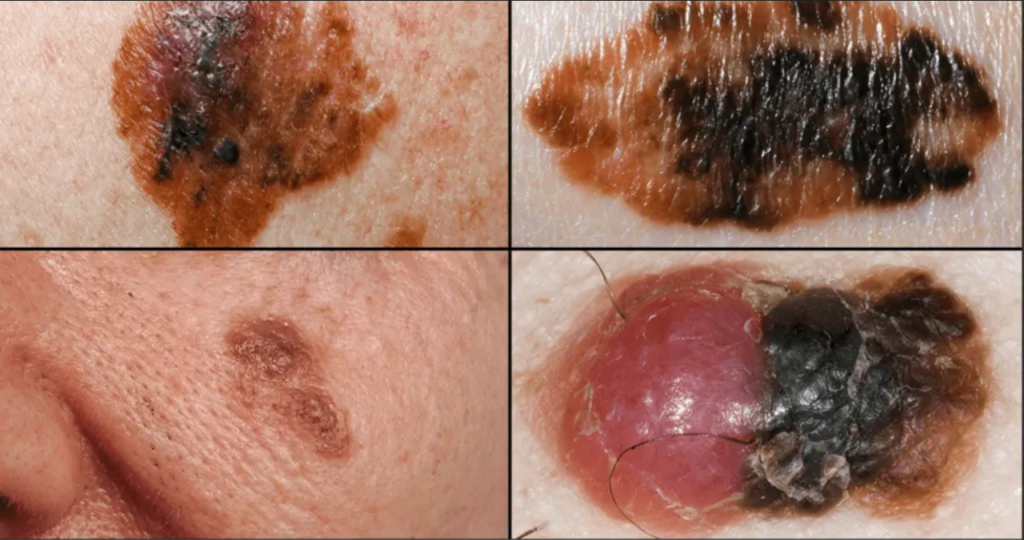
Reflecting on his participation in the trial, Young expressed, “[The trial] gave me a sense of actively combating a potential unseen threat. Despite being radiologically clear, there’s still the risk of undetected cancer cells. Rather than passively waiting, this trial allows me to take proactive measures.” He emphasized the importance of early detection, given his personal experience with melanoma.
Phase II trial data, published in December, revealed promising outcomes, with participants who received the vaccine alongside Keytruda showing significantly lower rates of cancer recurrence or mortality.
Dr. Shaw expressed optimism about the therapy’s potential as a “game-changer,” highlighting its relatively tolerable side effects, which include fatigue and mild arm soreness. She reassured me that for most patients, these side effects are comparable to those experienced with routine vaccines.