The examination of human brain tissue revealed distinct behaviours of immune cells in brains afflicted with Alzheimer’s disease, presenting a promising avenue for new treatment approaches. Research spearheaded by the University of Washington, unveiled in August, unearthed that microglia in Alzheimer ‘s-affected brains tended to exist in a pre-inflammatory state more frequently, indicating diminished protective capabilities.
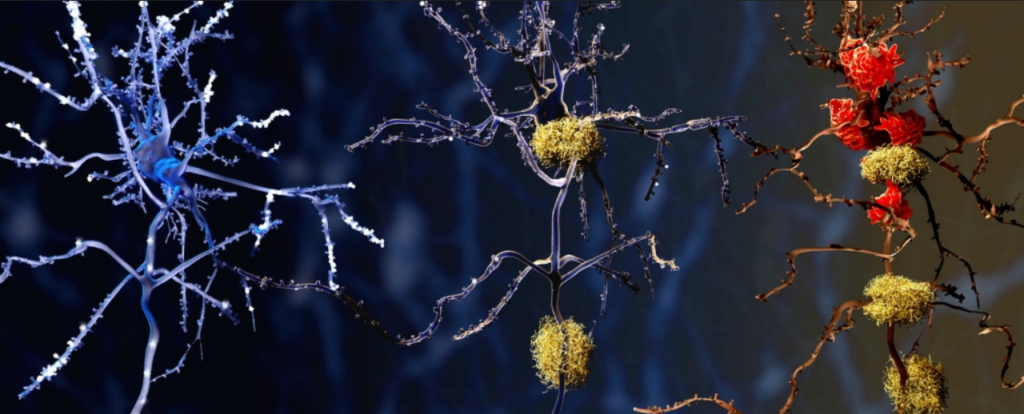
Microglia, crucial immune cells responsible for maintaining brain health by clearing debris and upholding normal function, exhibit adaptability in response to infection or cell clearance, transitioning to a more agile form to engulf and eliminate invaders. They also play a crucial role in sculpting brain circuitry by “pruning” synapses during development.
Although their precise involvement in Alzheimer’s remains somewhat enigmatic, in individuals afflicted with this debilitating neurodegenerative disorder, certain microglia exhibit an overly robust response, potentially instigating inflammation that contributes to neuronal demise. Regrettably, clinical trials evaluating anti-inflammatory medications for Alzheimer’s have yielded limited efficacy.
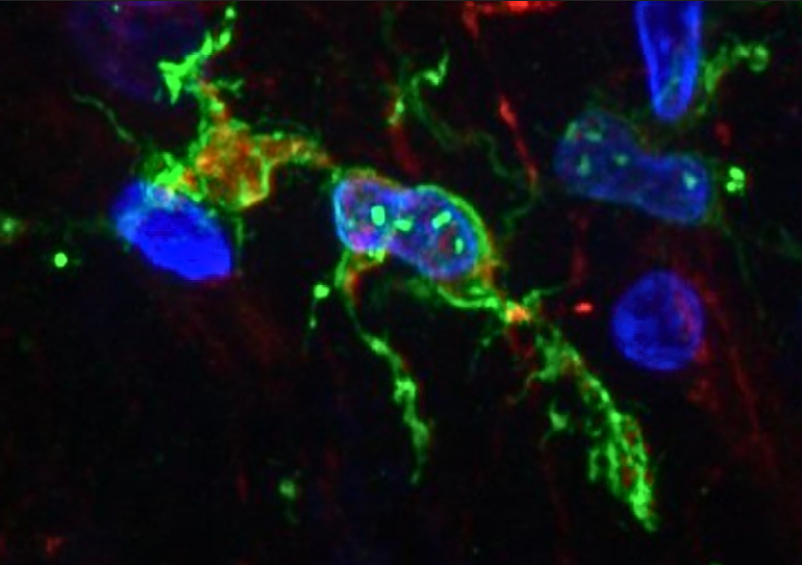
In an effort to illuminate the role of microglia in Alzheimer’s disease, neuroscientists Katherine Prater and Kevin Green from the University of Washington, alongside collaborators from various US institutions, scrutinized brain autopsy samples from research donors. The study encompassed 12 individuals with Alzheimer’s and 10 healthy counterparts, focusing on the gene activity of microglia.
Leveraging an innovative method to enhance single-nucleus RNA sequencing, the team discerned ten distinct microglia clusters within brain tissue, characterized by unique gene expression profiles governing cellular functions. Of note, three clusters had not been previously identified, with one particular cluster exhibiting higher prevalence in individuals with Alzheimer’s disease. This subset of microglia displayed heightened expression of genes implicated in inflammation and cell death.
Overall, the researchers observed a propensity for microglia clusters in Alzheimer ‘s-affected brains to adopt a pre-inflammatory state, signifying an elevated likelihood of producing inflammatory molecules that could inflict damage on brain cells and potentially exacerbate disease progression. Moreover, the microglia profiles in Alzheimer’s-affected brains appeared less conducive to protective functions, undermining their capacity to effectively clear cellular debris and promote healthy brain aging.
The researchers postulate that microglia may undergo phenotypic changes over time. Consequently, a comprehensive understanding of how microglia evolve over the disease course could offer insights into their contributions to Alzheimer’s disease pathology.
“At this point, we can’t say whether the microglia are causing the pathology or whether the pathology is causing these microglia to alter their behavior,” said Prater.
While the investigation is still in its nascent stages, these findings advance our comprehension of microglial involvement in Alzheimer’s disease and suggest potential therapeutic targets within specific microglia clusters. The team remains optimistic that their research will pave the way for the development of novel therapies aimed at ameliorating the burden of Alzheimer’s disease.
“Now that we have determined the genetic profiles of these microglia, we can try to find out exactly what they are doing and hopefully identify ways to change their behaviors that may be contributing to Alzheimer’s disease,” Prater said.
“If we can determine what they are doing, we might be able to change their behavior with treatments that might prevent or slow this disease.”
The study findings have been published in Nature Aging.